ApiFix Long Term Data: What We Are Doing in the Meantime
Understanding the Device
Introduction
New things can be both exciting and scary— I felt that way on my first day of college. Moving over 500 miles away from home was a big step, but my parents and I felt reassured by the research about my university. Though it was not the biggest or most well-known, its academics and job placement measured up to top schools. I can now say, from my experience, those statistics are happily true.
All that to say, choosing a life changing treatment like ApiFix can also be both nerve-wracking and exciting. It is important to us that you have a newfound freedom without scoliosis pain. And that the device used to do that is safe, smart, and works as intended.
While we cannot provide long term data on our device at this time, simply because enough time has not passed, we are doing everything we can at ApiFix while we wait to ensure the effectiveness of our product. But you can believe once that long-term day arrives, we will be eating it up faster than a college student at a campus event with free food!
Overview and Features
In case you missed it from our other content, I’ll review some key ApiFix features. The ApiFix device is a treatment for Adolescent Idiopathic Scoliosis that uses three screws and an extending rod with polyaxial joints (They function like a ball and socket joint). This type of rod works best on patients with a single curve in their spine creating a “C” shape. It is also recommended that a patient’s curve is relatively flexible for ApiFix to function appropriately.
Our device is FDA approved and also classified as a breakthrough device. That status means we work hard to gather as much data as possible from patients and surgeons to support the credibility of our product. While having long term results is not possible yet, we are working hard to plan for what ApiFix outcomes could be in the years to come through testing and comprehensive reviews of our ongoing data collection. That seemed more effective than getting our long-term results by building a time machine anyways.
Research and Clinical Trials
When I work on ApiFix’s socials, I can always count on at least one post every month. That is because a handy report dashboard of our US patients outcomes is compiled monthly for our team, surgeons, and partners. We have been gathering information like this on cases worldwide for the last 12 years. Nearly every patient’s data has been closely evaluated. This research along with scientific studies helps us refine the ApiFix design.
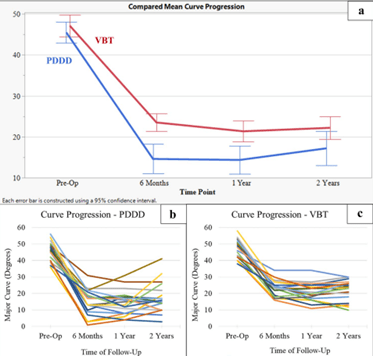
We know what you are wondering; how does this measure up to other non-fusion options out there like tethering systems? You are in luck, recently an article was published in 2024 comparing ApiFix to tethering systems over a two year period with 40 matched patients (20 for each device). ApiFix was found to “[demonstrate] better index correction, reduced operative time, less blood loss, and shorter length of [hospital] stay but higher rates of revision compared to a matched cohort of [tethering] patients at two-year follow-up” . This means that the procedure itself was less invasive, and recovery time was also quicker.
The device’s correction of the patient’s scoliosis was also better for ApiFix over tethering. The “high revision rate” metric means more patients needed their surgery fixed compared to the tether patients. In this case, of those 3 patients, 1 had it removed after the implant broke, and 2 of them needed a new, bigger device because of spine growth.
As for testing our key feature, preserving motion, a 2024 study was done with 29 patients measuring their ability to bend sideways (lateral bending) and front to back (flexion-extension) before and after surgery. These patients all had scoliosis at the beginning of the study. The results found that motion was preserved by 30-33%, with an average of 11° of motion in the upper back and 10° of motion in the lower back .
Surgeon Testimonials
It is one thing to know the stats, but what are those with personal ApiFix experience saying? We discussed with two surgeons who are experienced with the device about their thoughts.
Surgeon Geoffrey Haft is one of the first physicians to use ApiFix for his patients. Although it was only recent technology when Dr. Haft began to use the device, he was impressed with the data and had high hopes for the future of scoliosis treatment. He says “I see [ApiFix] as something that is going to give families the option that makes you more functional, that maintains more motion, and requires a lot less surgery”.
Another surgeon, Dr. Samuel Bederman, comments how ApiFix is especially impactful for correcting scoliosis in the lumbar (lower) spine. If the lumbar spine is treated using traditional fusion, it can “put a lot of stress on the other levels above and below, particularly because of flexibility”. ApiFix allows that motion to be maintained and overall provides lower risk than a fusion surgery with less blood loss, less invasive methods, and a quick recovery period of 1-3 days in the hospital.
Patient Success Stories

Next, let’s hear from some patients who live with the device everyday. You can listen to their full stories here: https://apifix.com/testimonials/
Emma is a patient treated with ApiFix in highschool and diagnosed with scoliosis at age 13. She was also a competitive swimmer and athlete. Her scoliosis journey began with bracing and then, after her curve progressed, she needed to receive surgery. As an athlete, she wanted to keep motion in her spine. Her diagnosis matched the ideal ApiFix patient requirements and it allowed her to maintain the flexibility needed for her active lifestyle.
In her patient testimonial video, her dad recounts asking surgeon Dr. Ron El-Hawary, “If this were your daughter, would you have her go through with the surgery?”. Dr. El-Hawary responded “he would”. That conversation assured the family that ApiFix was the right option for their daughter. Today, Emma has been post-ApiFix for years and she says receiving the device was “the best decision [she] ever made”.
Another patient, Danica (pictured here), received ApiFix as a university student. She was struggling with pain from scoliosis and felt limited in her physical capabilities because of her spine. Dr. Bederman, the surgeon who treated Danica, remarked that she was a patient who had more flexibility. This, along with her fear of fusion surgery and losing motion, lead her to choose ApiFix.
After treatment, she was walking the first day post-op. Life became more accessible for Danica with ApiFix, and she describes being able to now lift things normally and sit or sleep anywhere she wants without pain. One year after surgery, she says “it helped change my life… I just feel free, honestly”.
Addressing Long-Term Concerns
Over 900 patients have chosen ApiFix worldwide and we use the information from patient data to inform our product and practice. Currently the implant is in the 4th phase of development and real-world usage data is reviewed monthly.
While we acknowledge the lack of long-term data, we are actively monitoring and improving the device based on real-world feedback. This includes our successes and shortcomings. We are aware of previous studies that reported challenges and have, in response, made several design changes to enhance safety and effectiveness. Continuous updates to our surgical techniques ensure better patient outcomes.Our patient curve diagnosis qualifications have also been changed to ensure safe function of the engineering.
Conclusion
While ApiFix may not have long term data on the current, improved version, while waiting for years to pass, ApiFix is anything but passive! Since time travel isn’t an option yet, instead we have been studying and testing the device, improving the design and methods, and seeking feedback from surgeons and patients about their experience with us.
You can find the testimonial videos of all the patients and surgeons mentioned here on our website, Apifix.com. There you will also find resources like our free ebook, blog, and links to the clinical trials and studies referenced in this article. You can also check out our email newsletter to receive updates and information on all things ApiFix.
As Dr. El-Hawary remarks in his testimonial video: “Within the first 10 years of my practice the only surgical option we had was really fusion and I’ve always known in my heart that we can do better than that”. That is what we strive for, to provide a new option for AIS patients that allows them to keep doing what they love even after curve correction. We are confident the future of scoliosis is bright and it is a privilege to be even a small part of that journey together.
Resources
ApiFix. “ApiFix | New Treatment Option | Adolescent Idiopathic Scoliosis.” Accessed April 9, 2024. https://apifix.com/home/.
ApiFix. ApiFix Patient Story, 2023. https://www.youtube.com/watch?v=PH73bBpqwSg.
———. Surgeon Testimonial – Dr. Geoffrey Haft, 2021. https://www.youtube.com/watch?v=0ZwBjH1O0qo.
———. Surgeon Testimonial – Dr. Ron El-Hawary, 2023. https://www.youtube.com/watch?v=vtyWevGR43w.
———. Surgeon Testimonial – Dr. Samuel Bederman, 2024. https://www.youtube.com/watch?v=hwLgmx0TQzE.
ApiFix Animation. Accessed April 9, 2024. https://www.youtube.com/watch?v=4c2hu9pDE8I.
Arnin, U., R. El-Hawary, R. R. Betz, B. S. Lonner, and Y. Floman. “Preclinical Bench Testing on a Novel Posterior Dynamic Deformity Correction Device for Scoliosis.” Spine Deformity 7, no. 2 (March 1, 2019): 203–12. https://doi.org/10.1016/j.jspd.2018.08.010.
Danica Patient Story. Accessed April 9, 2024. https://www.youtube.com/watch?v=KAdVHcikJoY.
Froehlich, Susanne, Wolfram Mittelmeier, Biren Desai, Subash Jung Pandey, Herbert Raddatz, Bjoern Lembcke, Annett Klinder, and Katrin Osmanski-Zenk. “Surgical Treatment of Adolescent Idiopathic Scoliosis with the ApiFix Minimal Invasive Dynamic Correction System—A Preliminary Report of a 24-Month Follow-Up.” Life 13, no. 10 (October 2023): 2032. https://doi.org/10.3390/life13102032.
Holewijn, Roderick M., Marinus De Kleuver, Albert J. Van Der Veen, Kaj S. Emanuel, Arno Bisschop, Agnita Stadhouder, Barend J. Van Royen, and Idsart Kingma. “A Novel Spinal Implant for Fusionless Scoliosis Correction: A Biomechanical Analysis of the Motion Preserving Properties of a Posterior Periapical Concave Distraction Device.” Global Spine Journal 7, no. 5 (August 2017): 400–409. https://doi.org/10.1177/2192568217699377.
Richard, Olivia Kristina, Aléthéa Liens, DesiRae Muirhead, and Klaus Weber. “Tissue Response Following Implantation with the Posterior Dynamic Distraction Device (PDDD) in Adolescent Idiopathic Scoliosis (AIS).” European Spine Journal, April 8, 2024. https://doi.org/10.1007/s00586-024-08200-1.
Stadhouder, Agnita, Roderick M. Holewijn, Tsjitske M. Haanstra, Barend J. van Royen, Moyo C. Kruyt, and Marinus de Kleuver. “High Failure Rates of a Unilateral Posterior Peri-Apical Distraction Device (ApiFix) for Fusionless Treatment of Adolescent Idiopathic Scoliosis.” The Journal of Bone and Joint Surgery. American Volume 103, no. 19 (October 6, 2021): 1834–43. https://doi.org/10.2106/JBJS.20.02176.
Todderud, Julia E., Todd A. Milbrandt, Edward Floyd, Geoffrey Haft, Ron El-Hawary, Michael Albert, and A. Noelle Larson. “Preliminary Study of Motion Preservation Following Posterior Dynamic Distraction Device in Adolescent Idiopathic Scoliosis Patients.” Journal of Pediatric Orthopaedics 44, no. 9 (October 2024): 524–29. https://doi.org/10.1097/BPO.0000000000002739.
Todderud, Julia, A. Noelle Larson, Geoffrey Haft, Ron El-Hawary, Nigel Price, John T. Anderson, Ryan Fitzgerald, et al. “Matched Comparison of Non-Fusion Surgeries for Adolescent Idiopathic Scoliosis: Posterior Dynamic Distraction Device and Vertebral Body Tethering.” Spine Deformity, October 8, 2024. https://doi.org/10.1007/s43390-024-00982-0.
 English
English Español
Español Deutsch
Deutsch

